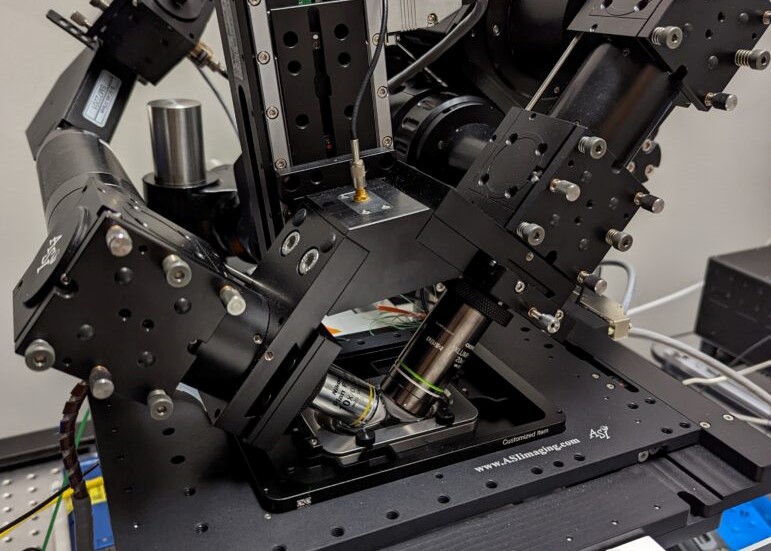
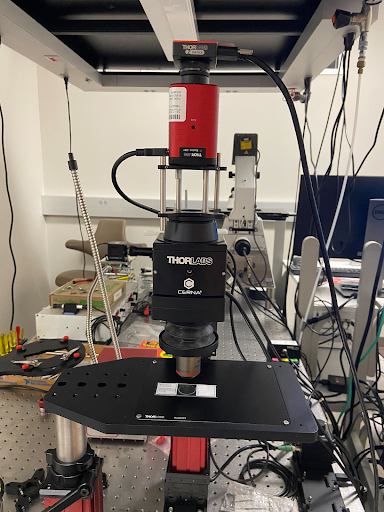
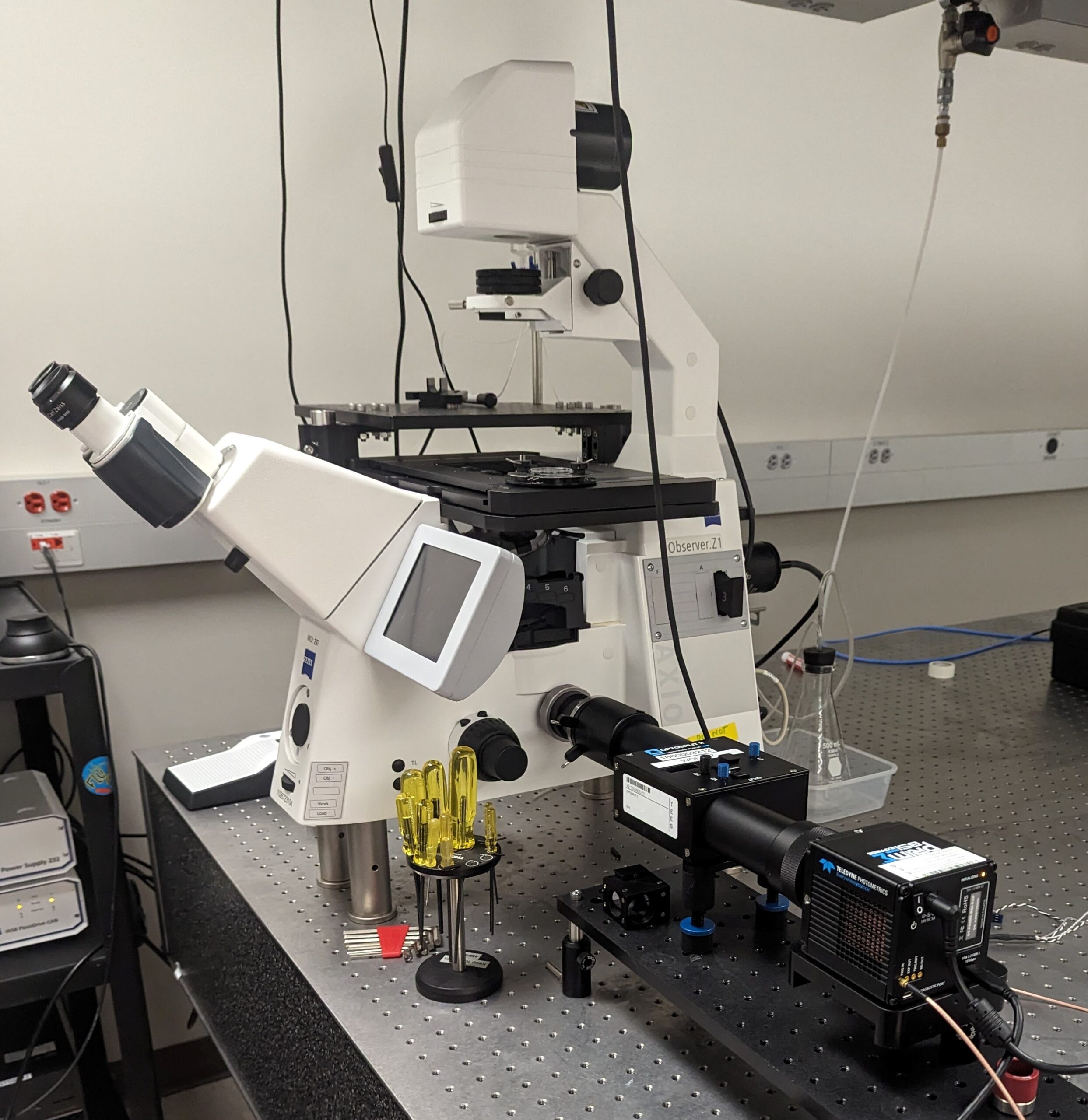
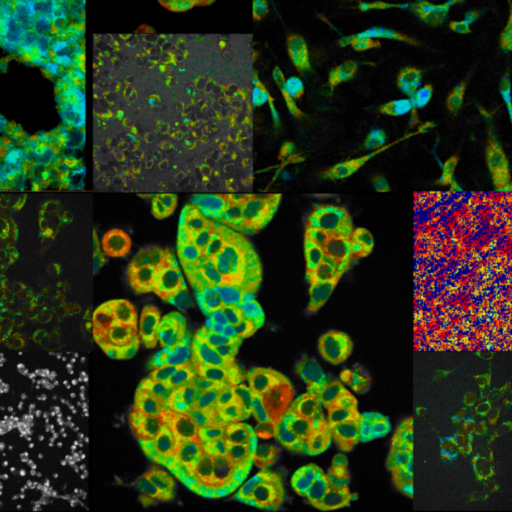
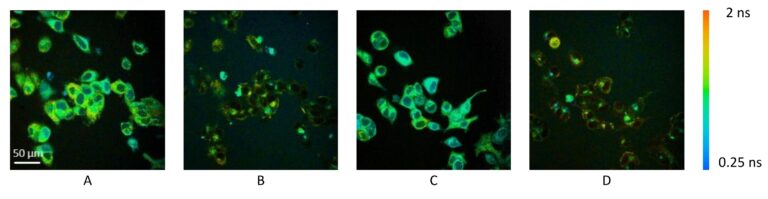
Multiphoton Fluorescence Lifetime Microscope
Multiphoton fluorescence lifetime microscope with tunable TiSapph laser (~680-1080 nm) for excitation. Two channel FLIM imaging plus a third intensity PMT. Inverted configuration with 20X, 0.8 NA air objective, 40X 1.1 NA water objective, and 100X 1.46 NA oil objective. Emission filters include 447/60 nm, 550/88 nm, and 675/67 nm. Environmental control for live cell and in vivo imaging.