Our Mission: To improve human health through the development and application of label-free optical technologies.
Our Technologies
Lightsheet Microscopy
In lightsheet microscopy, the illumination light is focused into a plane and emission or reflected light from the plane is captured on a camera. Lightsheet microscopy has high axial (depth) resolution and is fast.
Autofluorescence Imaging
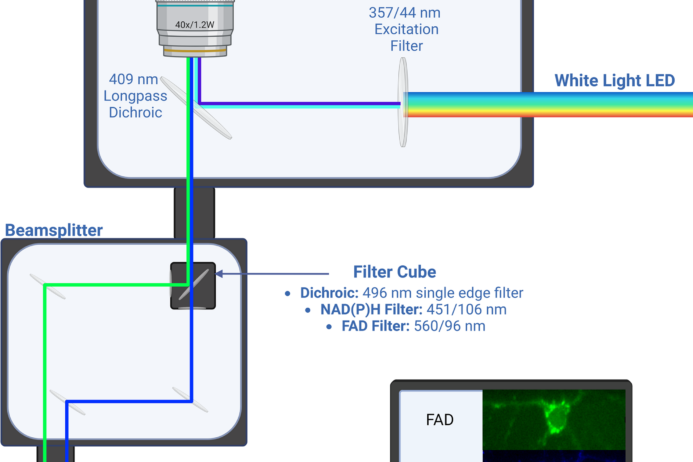
Several endogenous molecules including the metabolic coenzymes NADH and FAD have fluorescence properties. Autofluorescence imaging uses these molecules that are already within cells and tissues for image contrast.
Machine Learning for Image Analysis
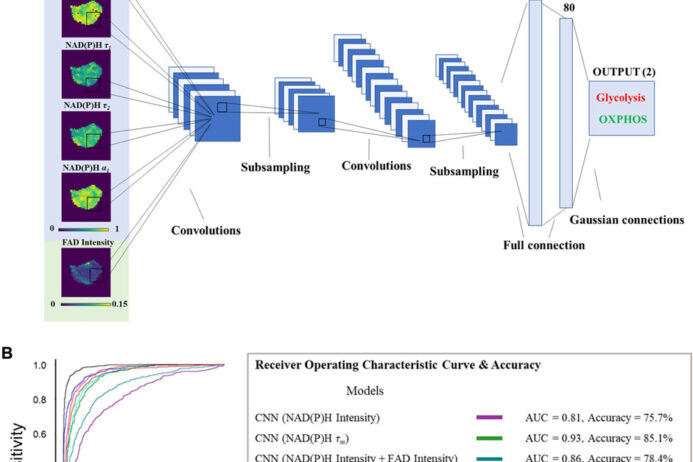
Machine learning allows for efficient image segmentation, de-noising, and classification.
Fluorescence Lifetime Imaging
The fluorescence lifetime is the time a fluorophore remains in the excited state before returning to the ground state, and is on the order of hundreds of picoseconds to several nanoseconds. The fluorescence lifetime provides molecular information (such as conformation, pH, temperature, viscosity, etc) about the fluorophore and environment.
Infrared Stimulation
Infrared stimulation is the excitation or inhibition of neural activity with short pulses of infrared light (~1450-1870 nm). Infrared stimulation does not require chemical or genetic manipulation. Although the exact mechanism of infrared stimulation is under study, it is dependent on a fast thermal gradient.
Hyperspectral Imaging
Hyperspectral imaging acquires many images across a wavelength spectrum, with each image containing a narrow-bandwidth of light. Hyperspectral imaging can be used with absorption/diffuse reflectance imaging to quantify tissue perfusion, oxygenation, and optical properties.
Selected Projects
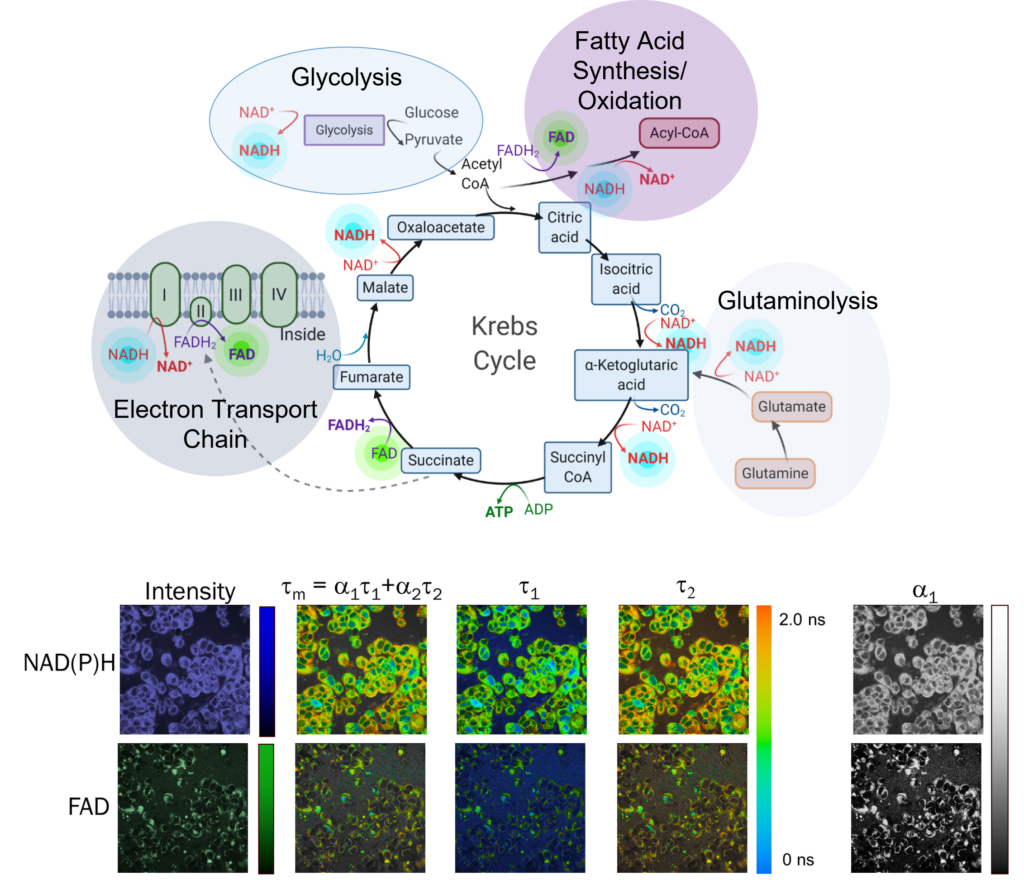
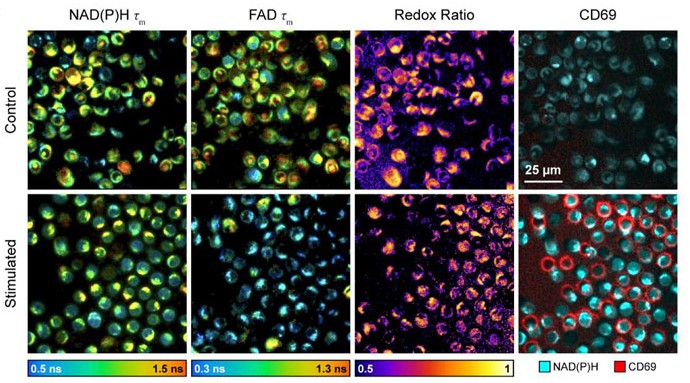
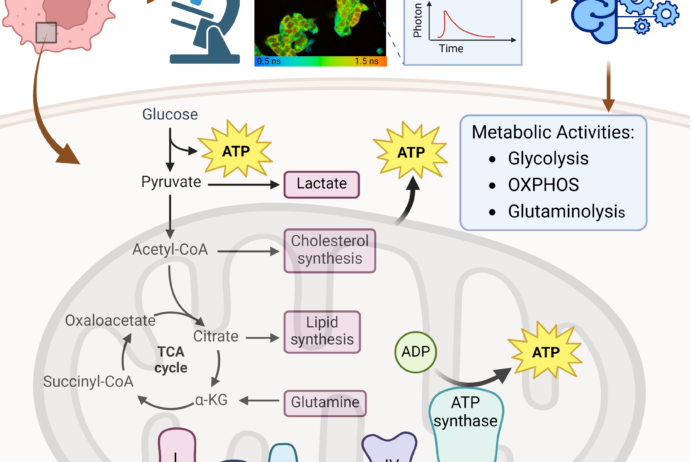
Reduced nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD) are two co-enzymes that are used in metabolic reactions. These molecules, NADH and FAD, are fluorescent which enables label-free imaging these molecules on fluorescence microscopes. The fluorescence intensity and lifetime of NADH and FAD provide information about these molecules and their use in cells. We are currently working to develop new imaging methods for capturing metabolic dynamics within seconds, imaging mitochondria structure and movement concurrently with function, and improving specificity of metabolic interpretations from autofluorescence lifetime data.
Recent Publications:
- Autofluorescence Imaging to Evaluate Cellular Metabolism. Anna Theodossiou, Linghao Hu, Nianchao Wang, Uyen Nguyen, Alex J. Walsh, J. Vis. Exp. (177), e63282.
- Osr1 regulates macrophage-mediated liver inflammation in non-alcoholic fatty liver disease progression, Liu L, Liu Z, Zhou Y, Li J, Hu Linghao, He L, Gao G, Kidd B, Alex J Walsh, Jiang R, Wu C, Zhang K, Xie L, Cellular and Molecular Gastroenterology and Hepatology, 2022, 15(5) 1117-1133.
Short pulses (ms to ms) of infrared light (1400-2100 nm) can excite action potentials in neurons; however, the mechanism driving this phenomenon is unknown. We are currently testing the hypothesis that infrared light alters the metabolic or redox balance of a cell. In support of our hypothesis, we have developed a model of infrared light and thermal dynamics on cell metabolism and enzyme binding properties. We are also using fluorescence lifetime imaging to reveal insights into infrared light effects enzyme binding dynamics and fast, wide-field fluorescence imaging to evaluate infrared light effects on cellular metabolism.
Recent Publications:
- Fluorescence intensity and lifetime redox ratios detect metabolic perturbations in T cells. Hu L, Wang N, Cardona E, and Walsh AJ, Biomedical Optics Express, 2020 (11)10: 5674-5688.
- Classification of T-cell activation via autofluorescence lifetime imaging. Walsh AJ*, Mueller KP, Tweed K, Jones I, Walsh CM, Piscopo NJ, Niemi NM, Pagliarini DJ, Saha K, Skala MC*, Nature Biomedical Engineering, 2020.
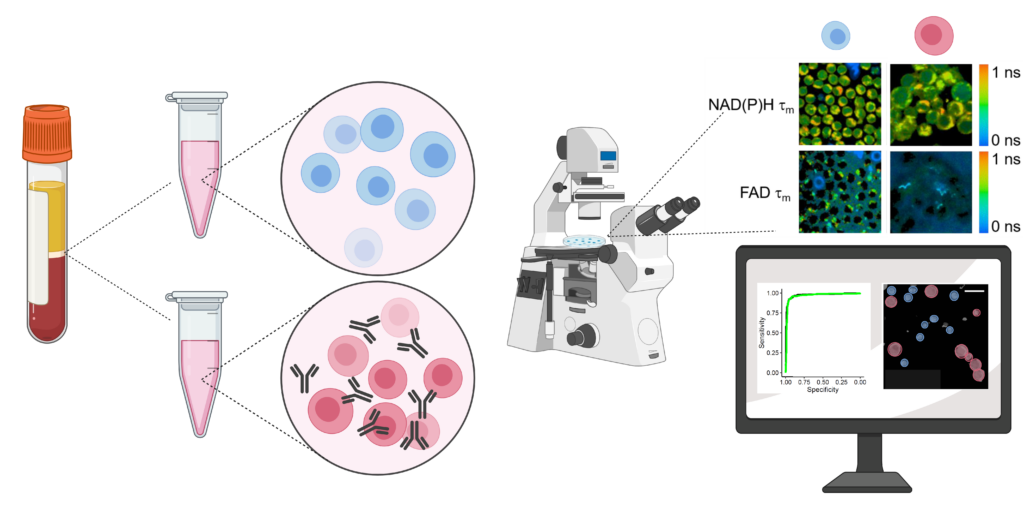
Currently, the activation of immune cells is assessed by label-based methods including flow cytometry and immunohistochemistry. Activated immune cells require high rates of glycolysis to maintain immune activities. Therefore, we are using fluorescence lifetime imaging of NAD(P)H and FAD which reports cellular metabolic information, to evaluate immune cell function. We have shown that the combination of machine learning classification of fluorescence imaging features achieves high accuracy (~98%) for classification of activation of T cells. Label-free imaging assays of immune cell function will allow unprecedented study of immune cell behaviors, functional assessment of immune cells in small volume samples (e.g. neonatal blood draws), and quality control/quality assurance of biomanufactured immune cells.
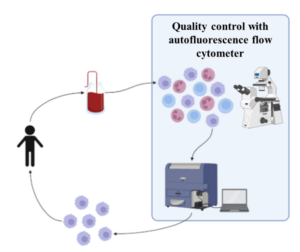
Cell-based therapies harness functional cells or tissues to mediate healing and treat disease. These cells or cell products must be cultured and manufactured in a manner that maintains sterility and cell viability and function. Currently, methods that can assess the viability and function of cells are destructive and/or reply on contrast agents that are not compatible for the use of the cells as a therapy. Optical imaging of endogenous collagen, by second-harmonic generation, and the metabolic coenzymes NADH and FAD, by autofluorescence microscopy, provides tissue structure and cellular information. We are working on optimizing label-free imaging systems and image analysis for continuous, in-line or on-line monitoring of biomanufactured cells.
Recent Publications:
- Volumetric imaging of human mesenchymal stem cells (hMSCs) for non-destructive quantification of 3D cell culture growth, Oscar R Benavides, Holly C Gibbs, BP White, Roland Kaunas, Carl A Gregory, Alex J Walsh, Kristen C Maitland, PlosOne. 2023. 18(3): e0282298.
- Label-free optical imaging of cell function and collagen structure for cell-based therapies, Linghao Hu, Samantha Morganti, Uyen Nguyen, Oscar Benavides, Alex J Walsh, Current Opinion in Biomedical Engineering, 2022.
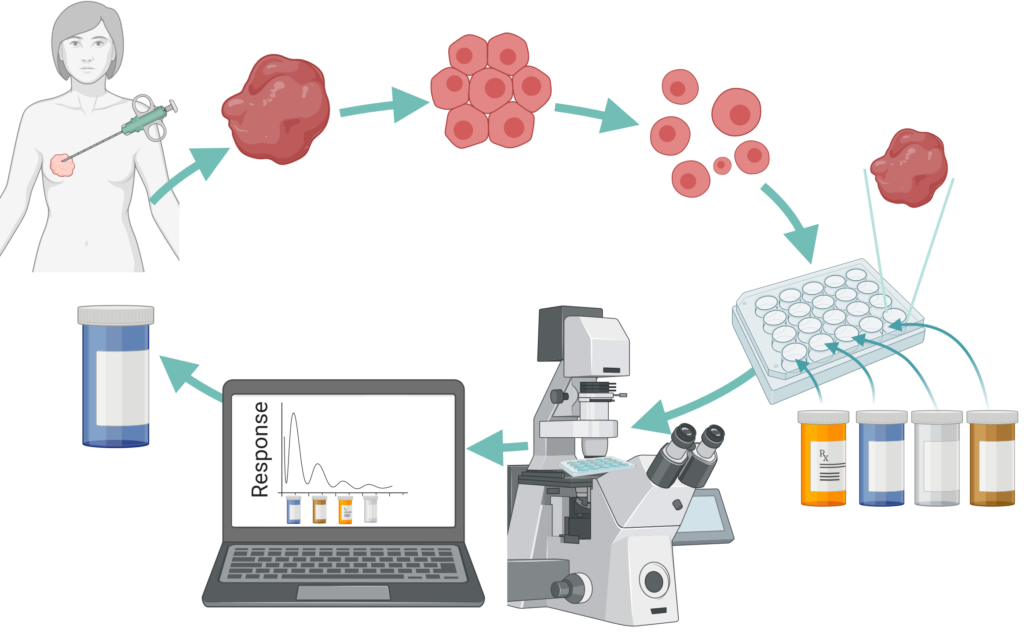
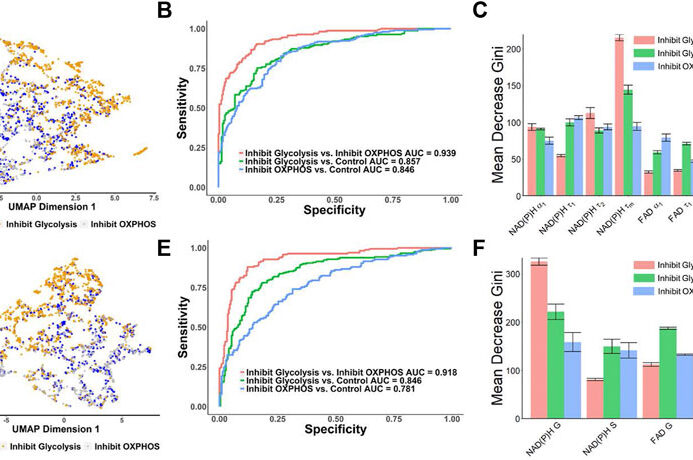
Tumor drug resistance due to heterogeneous cell populations remains a significant challenge for cancer treatment. Optical imaging of the fluorescence lifetimes of NADH and FAD, coenzymes of metabolism, can be used to track anti-cancer drug response in a label-free, non-contact manner. This imaging technique, “optical metabolic imaging,” can be performed on cancer cells and tumors to evaluate novel anti-cancer drugs or investigate mechanisms of resistance. Furthermore, multiphoton microscopy provides depth imaging of 3D samples such as patient derived primary tumor organoids. Optical metabolic imaging of organoids provides a predictive screen for individualized patient cancer treatment.
Optical metabolic microscopy can be combined with image segmentation for quantification of intra-population heterogeneity. Our work measuring and modeling cellular population dynamics reveals inherent and drug induced heterogeneity within cancer cells, providing a unique identification method of drug resistant cells.
Recent Papers:
- POSEA: A novel algorithm to evaluate the performance of multi-object instance image segmentation, Nianchao Wang, Linghao Hu, Alex J Walsh, PlosOne 18(3): e0283692.=, 2023.
- Evolution of cisplatin resistance through coordinated metabolic reprogramming of the cellular reductive state, Yu, W., Y. Chen, N. Putluri, A. Osman, C. Coarfa, V. Putluri, A. H. M. Kamal, J. K. Asmussen, P. Katsonis, J. N. Myers, S. Y. Lai, W. Lu, C. C. Stephan, R. T. Powell, F. M. Johnson, H. D. Skinner, J. Kazi, K. M. Ahmed, L. Hu, A. Threet, M. D. Meyer, J. A. Bankson, T. Wang, J. Davis, K. R. Parker, M. A. Harris, M. L. Baek, G. V. Echeverria, X. Qi, J. Wang, A. I. Frederick, A. J. Walsh, O. Lichtarge, M. J. Frederick and V. C. Sandulache, British Journal of Cancer, 2023.
- Identification of rare cell populations in autofluorescence lifetime image data. Elizabeth Cardona, Alex J. Walsh, Cytometry Part A, 2022.
- Autofluorescence Imaging to Evaluate Cellular Metabolism. Anna Theodossiou, Linghao Hu, Nianchao Wang, Uyen Nguyen, Alex J. Walsh, J. Vis. Exp. (177), e63282.
Collaborators
Shreya Raghavan, PhD
Biomedical Engineering
Texas A&M University
Phillip West, PhD
Microbial Pathogenesis & Immunology
Texas A&M Medicine
Nadezhda German, PhD
Pharmaceutical Sciences
Texas Tech University HSC
Feng Zhao, PhD
Biomedical Engineering
Texas A&M University
Peter Davies, MD, PhD
Institute of Biosciences and Technology
Texas A&M Health
Johannes Schoneberg, PhD
Pharmacology, Chemistry, & Biochemistry
UC San Diego
Adela Oliva Chavez, PhD
Entomology
Texas A&M University
Clifford Stephan, PhD
Institute of Biosciences and Technology
Texas A&M Health
Gloria Echeverria, PhD
Baylor College of Medicine
Vanna Dickerson, DVM
Small Animal Soft Tissue Surgery
Veterinary Medicine & Biomedical Sciences
Texas A&M University
Linglin Xie, PhD
Nutrition
Texas A&M University
Vlad Sandulache, MD, PhD
Otolaryngology-Head & Neck Surgery
Baylor College of Medicine
Matt Powell-Palm, PhD
Mechanical Engineering
Texas A&M University
Funding
Current Projects:
– FA9550-20-1-0078 (AFOSR): Optical modulation of cell redox state
– RP200668 (CPRIT): GCC Combinatorial Drug Discovery Program (CDDP)
– R35 GM142990 (NIH, NIGMS): Autofluorescence lifetime microscopy for label-free detection of cell metabolism for cell biology research
– 2022-251380 (CZI): Dynamic 4D imaging and tracking to resolve organelle form vs function
– R37CA269224 (NIH, NCI): 3D Engineered Model of Microscopic Colorectal Cancer Liver Metastasis for Adjuvant Chemotherapy Screens
Completed Projects:
– FA9550-21-1-0280 (AFOSR): (DURIP) Light-Sheet Microscope with Optical Modulation Unit for Research on Biophysical Effects of Infrared Radiation
– (TAMU) Longitudinal evaluation of macrophage immuno-modulation in 3D tumor organoids
– (TAMU) Design and implementation of an accessible OCT lab to enhance biophotonics education